Geovanna, a ZEVASKYN patient at age 8
Strong Bonds Stand the Test of Time
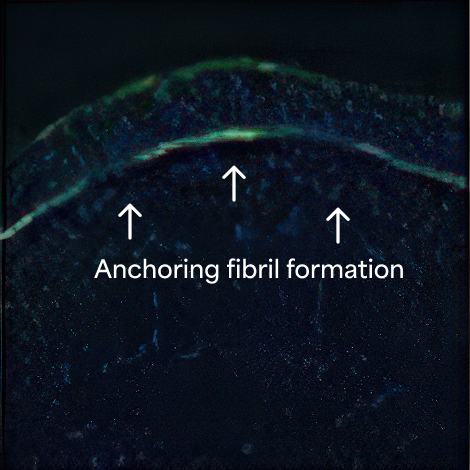
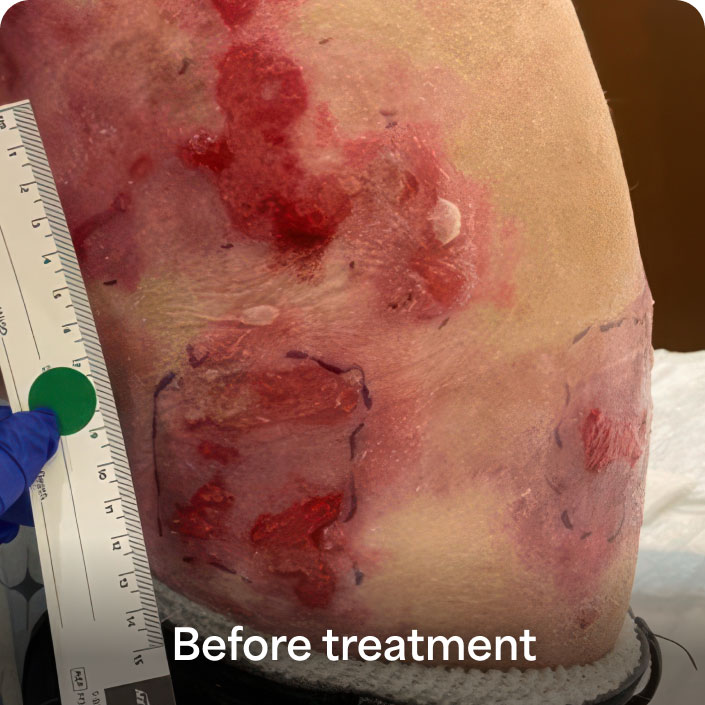
Durable wound healing with a single surgical application—even in tough RDEB wounds1*
- 81% of ZEVASKYN-treated wounds (35/43) achieved 50% or more healing vs 16% of matched control wounds (7/43) at week 24 in a Phase 3 study (P<0.0001)†
How ZEVASKYN works
Learn how patients’ own skin cells are gene-modified to create cellular sheets
Help patients connect
Through the Strong Together Network, learn about experiences with ZEVASKYN from patients living with RDEB and their caregivers
All-in-one support
From personalized help to comprehensive resources, Abeona Assist is here for your patients

When the nurse and doctor took off the bandage and I saw that new skin, when I saw it I just cried.”
- RDEB=recessive dystrophic epidermolysis bullosa.
- *Wounds treated had remained open for at least 6 months.
- †Investigator-assessed; multi-center, randomized, intrapatient-controlled study with 43 ZEVASKYN-treated wounds vs 43 matched control wounds in 11 patients with RDEB.
Reference: 1. ZEVASKYN (prademagene zamikeracel) Prescribing Information. Cleveland, OH: Abeona Therapeutics Inc; 2025.