ZEVASKYN (ZEE-vah-skin) is made from patients’ own skin cells that are genetically modified with copies of the functional COL7A1 gene1
- Autologous keratinocytes are isolated from skin punch biopsies, then transduced ex vivo with a retroviral vector containing the full-length COL7A1 gene
- The resulting gene-modified cellular sheets express functional type VII collagen (C7) protein
- Stable integration of the COL7A1 gene is maintained through cell division at the treated site even after ZEVASKYN application
- Expression of functional C7 results in the formation of anchoring fibrils (AFs)1,2
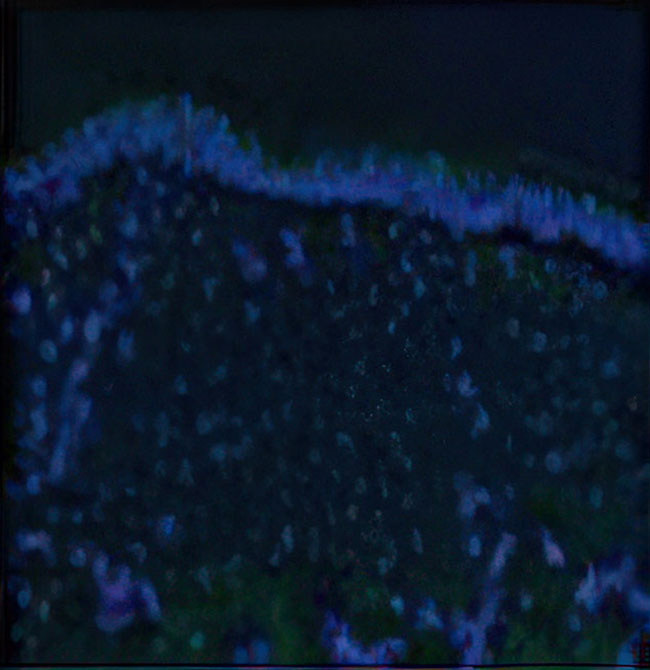
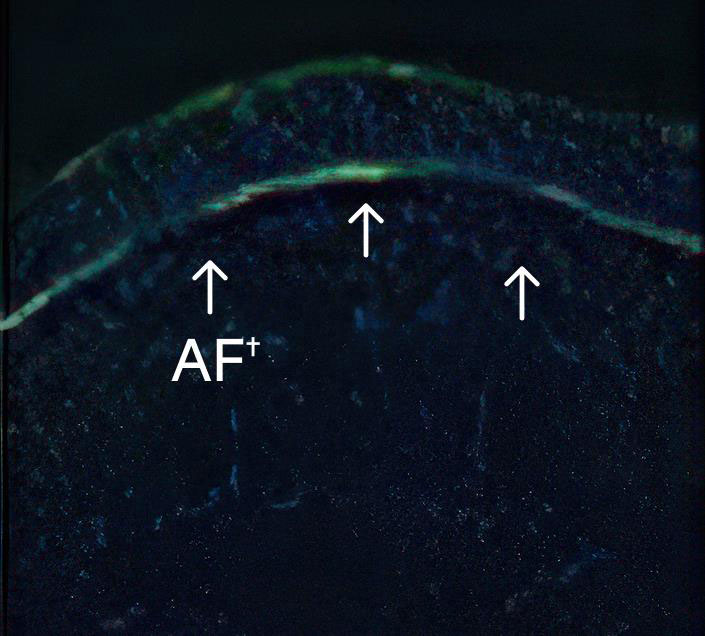
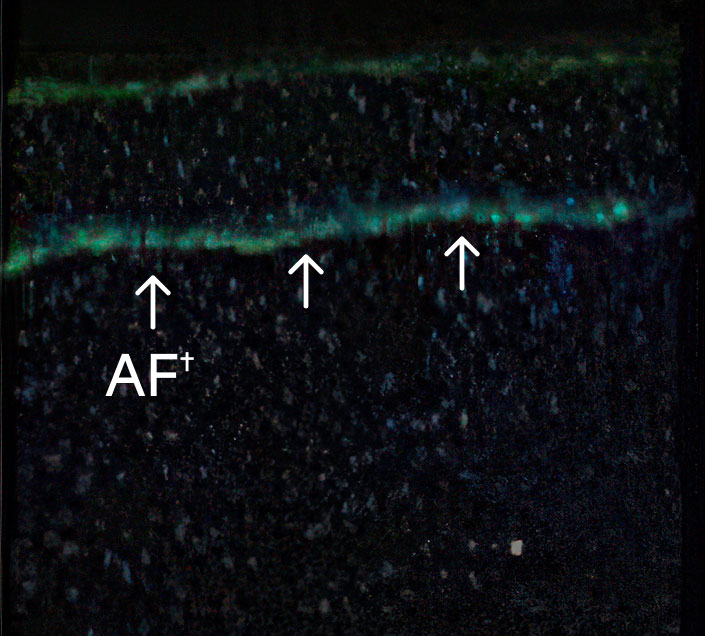
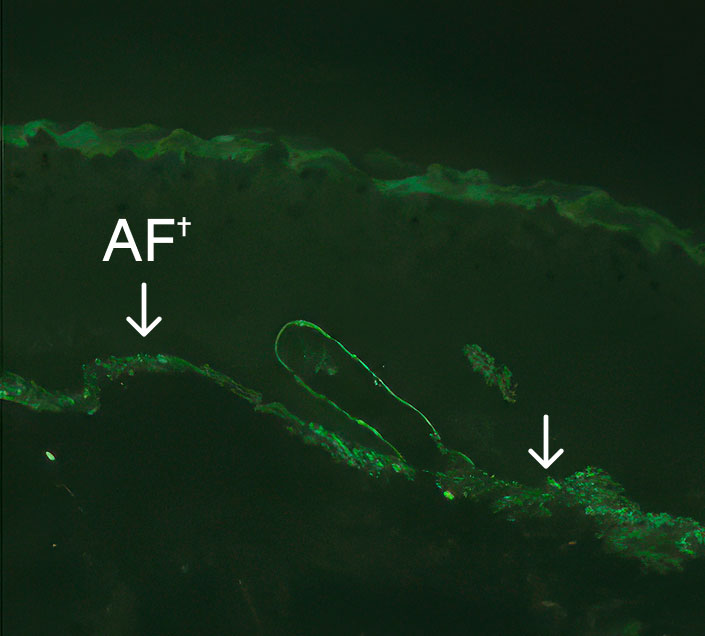
Long-term presence of C7 and anchoring fibrils at treated sites1
Baseline2*
3 months2*
12 months2*
24 months3*
- *Immunofluorescence images.
Patients treated with ZEVASKYN were assessed for presence of C7 or AFs.‡
- At 3 months, 86% of patients (6/7) were positive for both C7 and AFs
- At 6 months, 71% of patients (5/7) were positive for both C7 and AFs
- At 1 year, 43% of patients (3/7) were positive for C7 or AFs
- At 2 years, 67% of patients (2/3) were positive for C7 or AFs§
Assessment was elective and performed periodically because each test required a biopsy of healed skin.
- ‡In the Phase 1/2a study, C7 expression was assessed by immunofluorescence microscopy (negative cofactor 2 domain of C7 using the LH24 antibody) and anchoring fibrils were assessed by immunoelectron microscopy.
- §One patient was positive for both C7 and AFs; one patient was positive only for C7 (as biopsy to assess AF was not obtained).
References: 1. ZEVASKYN (prademagene zamikeracel) Prescribing Information. Cleveland, OH: Abeona Therapeutics Inc; 2025. 2. Siprashvili Z, Nguyen NT, Gorell ES, et al. JAMA. 2016;316(17):1808-1817. doi:10.1001/jama.2016.15588 3. Eichstadt S, Barriga M, Ponakala A, et al. JCI Insight. 2019;4(19):e130554. doi:10.1172/jci.insight.130554