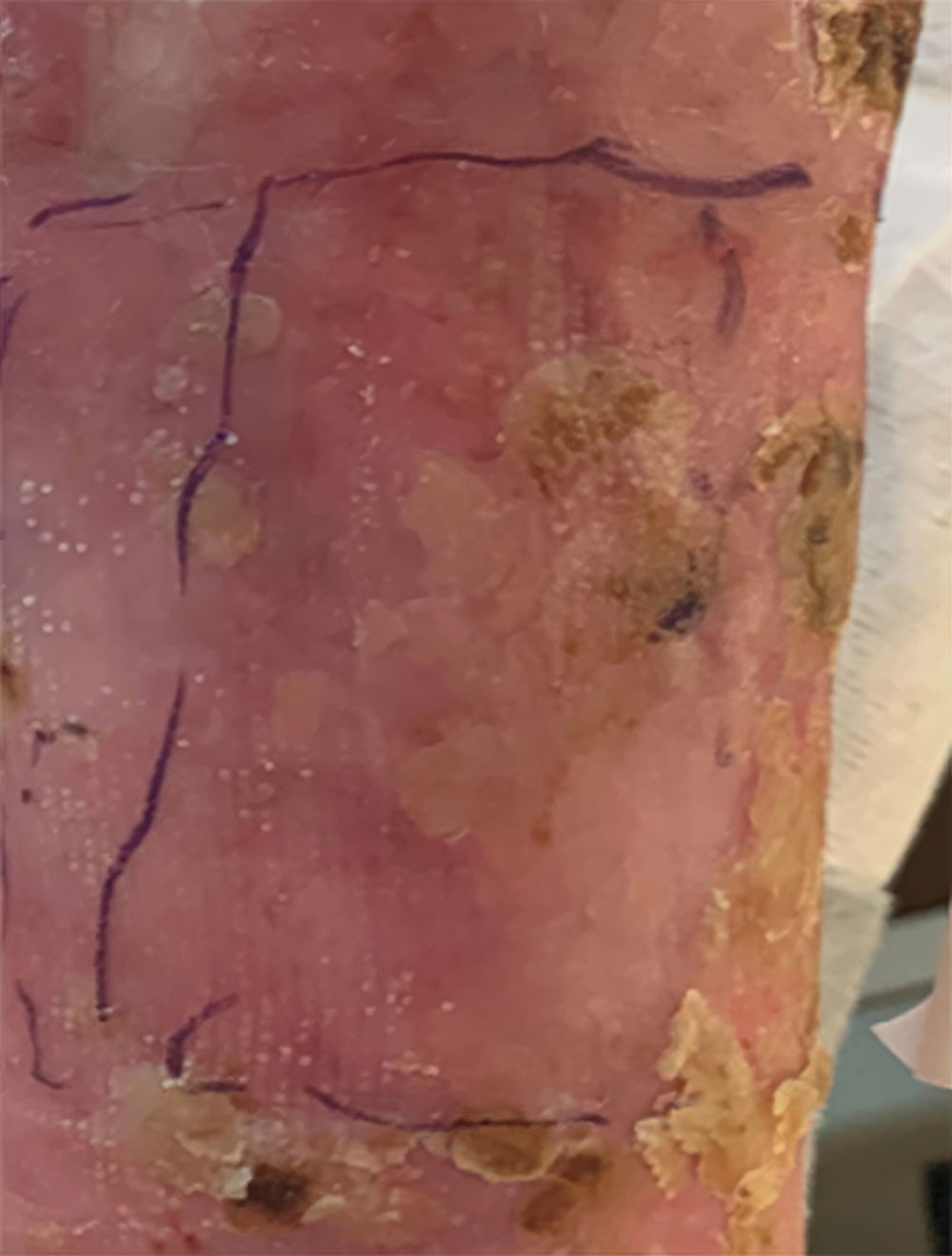Phase 3 (VIITAL study)
Phase 1/2a
- Study design
- Healing at week 24
- Before and after images
- Pain/itch reductions
- Long-term follow-up images
Phase 1/2a
- Long-term follow-up images
Phase 3 (VIITAL study) | Study Design
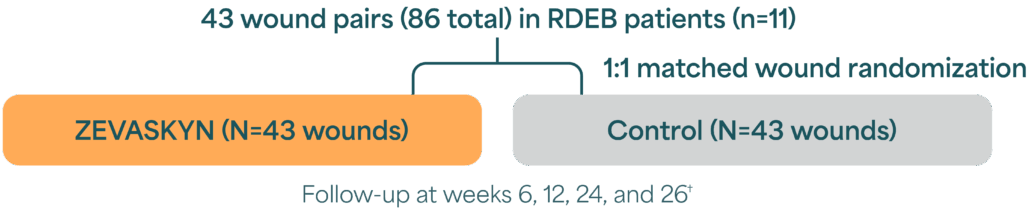
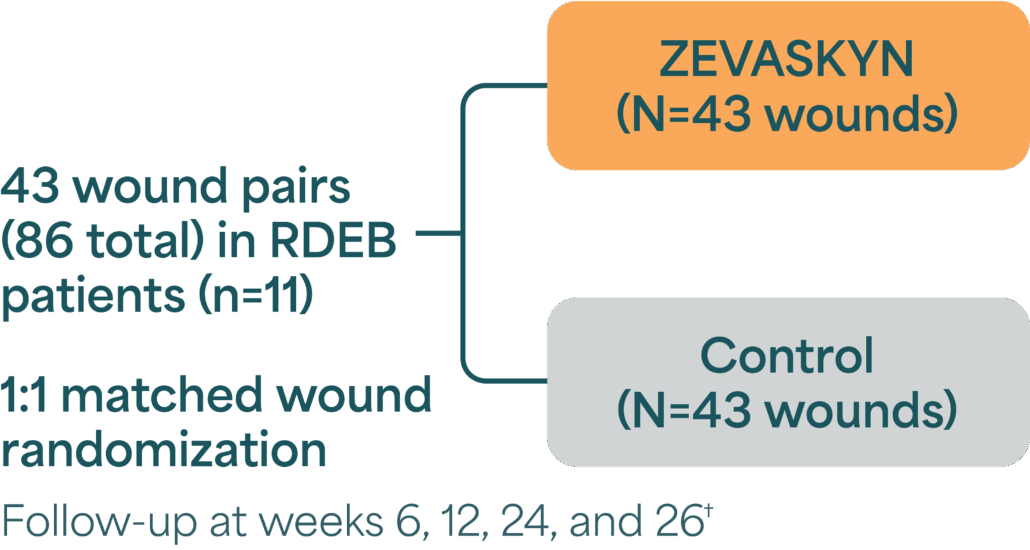
VIITAL Phase 3 study design
A randomized, intrapatient-controlled trial comparing ZEVASKYN with conventional wound management1,2*
Key endpoints (change from baseline at week 24)1,2
- ≥50% wound healing (coprimary)
- Pain reduction (coprimary)
- Complete wound healing (secondary)‡
- ≥75% wound healing (exploratory)
- Itch reduction (exploratory)
Select baseline demographics1,2
- Median patient age: 21 years (range 6-40 years)
- Median body surface area treated with ZEVASKYN: 240 cm2 (6 sheets)§
Select wound demographics1,2
- Each wound evaluated was ≥20 cm2
- Median wound duration: 5 years (range 6 months-21 years)
- N=wounds, n=patients.
- *Supportive care, such as daily bandaging and palliative measures.2
- †Wounds were assessed by investigator based on predefined criteria to score healing. Healing achieved at week 24 was confirmed at least 2 weeks later (week 26).1
- ‡Complete wound healing defined as complete re-epithelialization with no drainage or erosion, and no major crusting.1
- §ZEVASKYN was placed on 57 wounds (43 randomized and 14 non-randomized); range of body surface area treated: 120-240 cm2 (3-6 sheets).1,2
Phase 3 (VIITAL study) | Healing at week 24
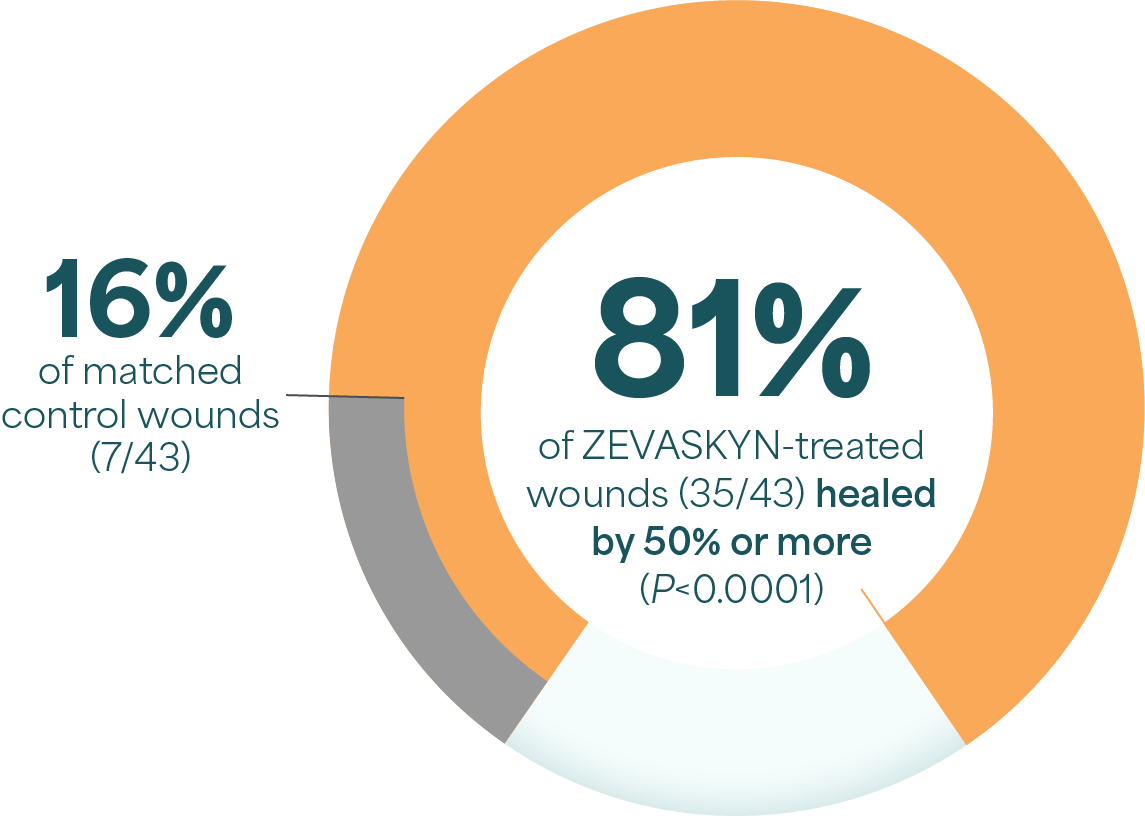
ZEVASKYN can close even tough RDEB wounds1
ZEVASKYN achieved significant healing as evaluated at week 241
- Complete healing achieved in 16% of ZEVASKYN-treated wounds (7/43) vs 0% of matched control wounds (0/43; P=0.0160)
- ~2 out of 3 ZEVASKYN-treated wounds (28/43) healed by 75% or more vs 7% of matched control wounds (3/43)2
In post hoc analysis of evaluated wounds at 6 weeks, 96% of ZEVASKYN-treated wounds (27/28) were healed by 50% or more vs 25% of control wounds (7/28)2||
- ||Missing data were not imputed; observed cases only.2
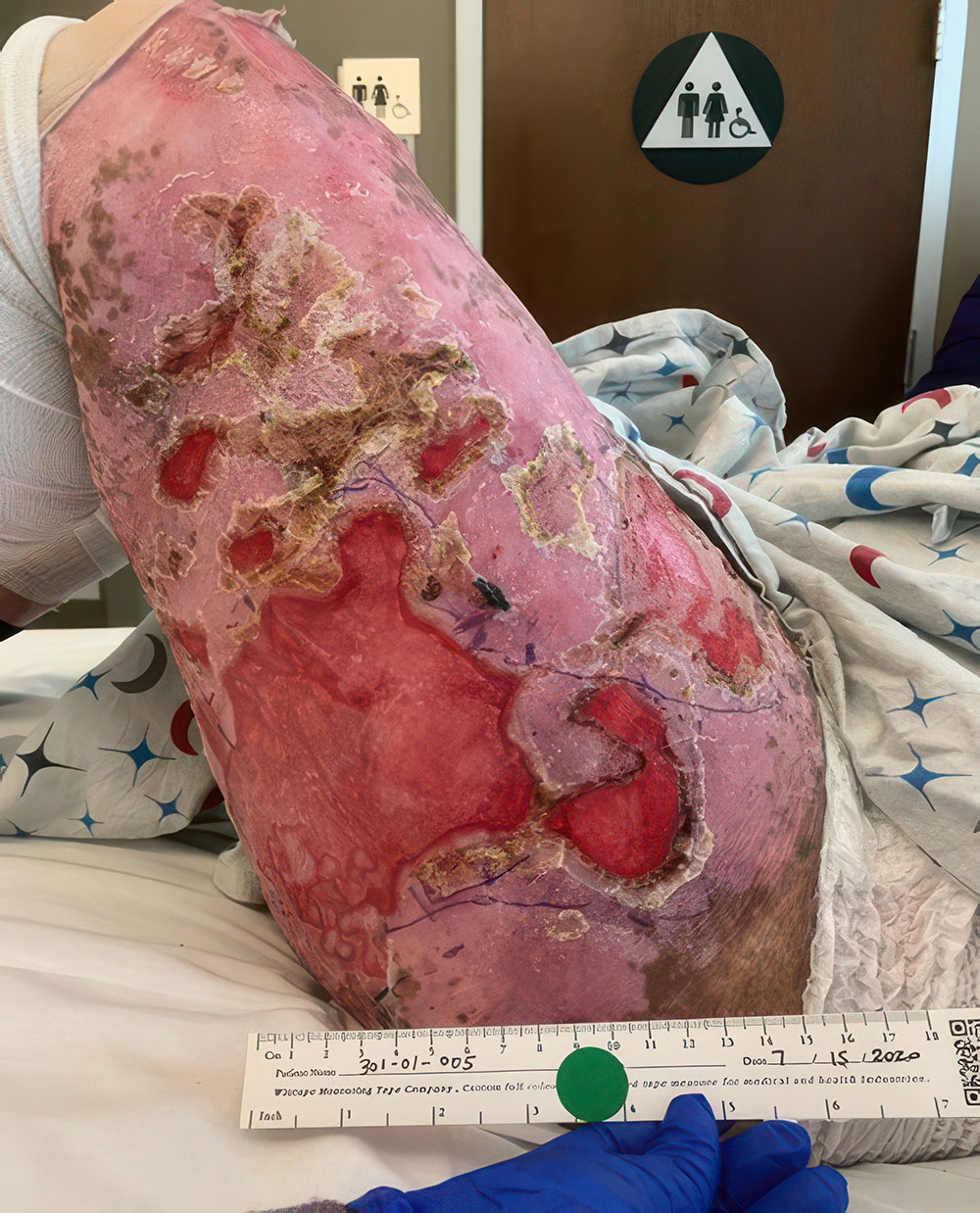
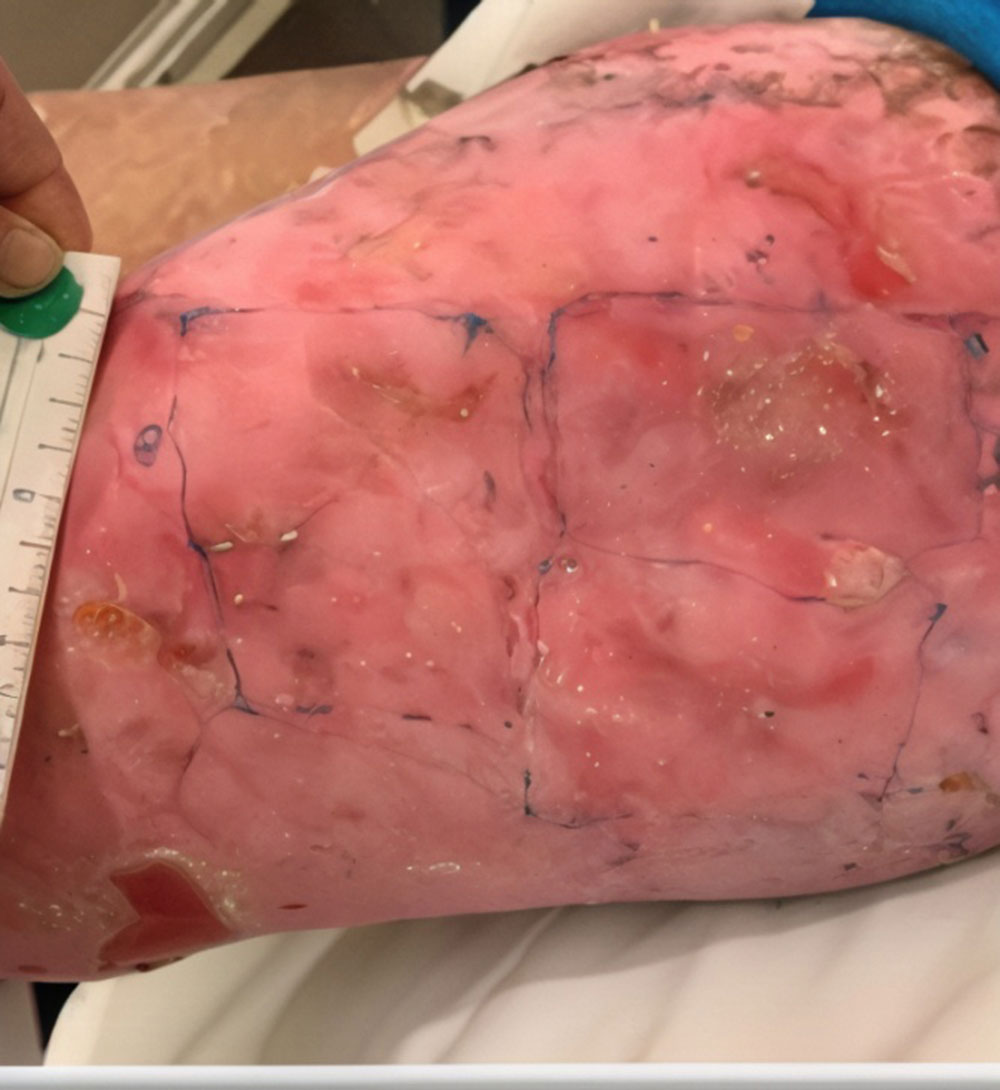
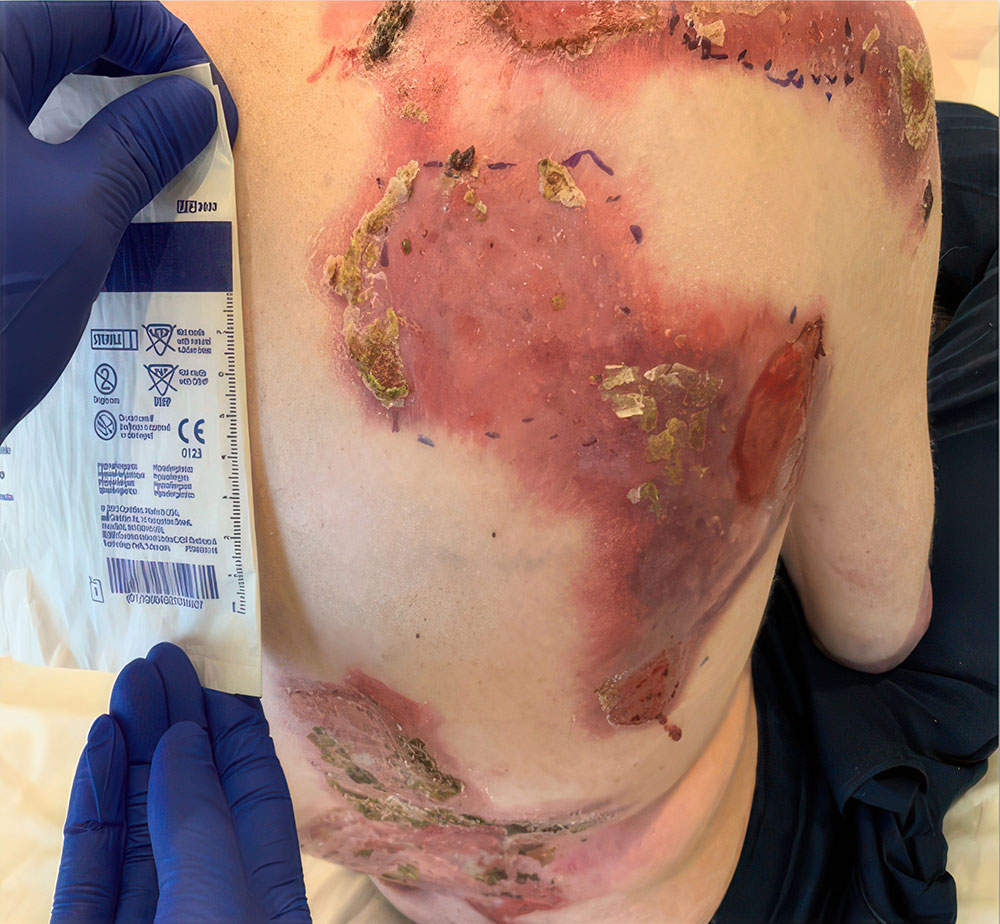
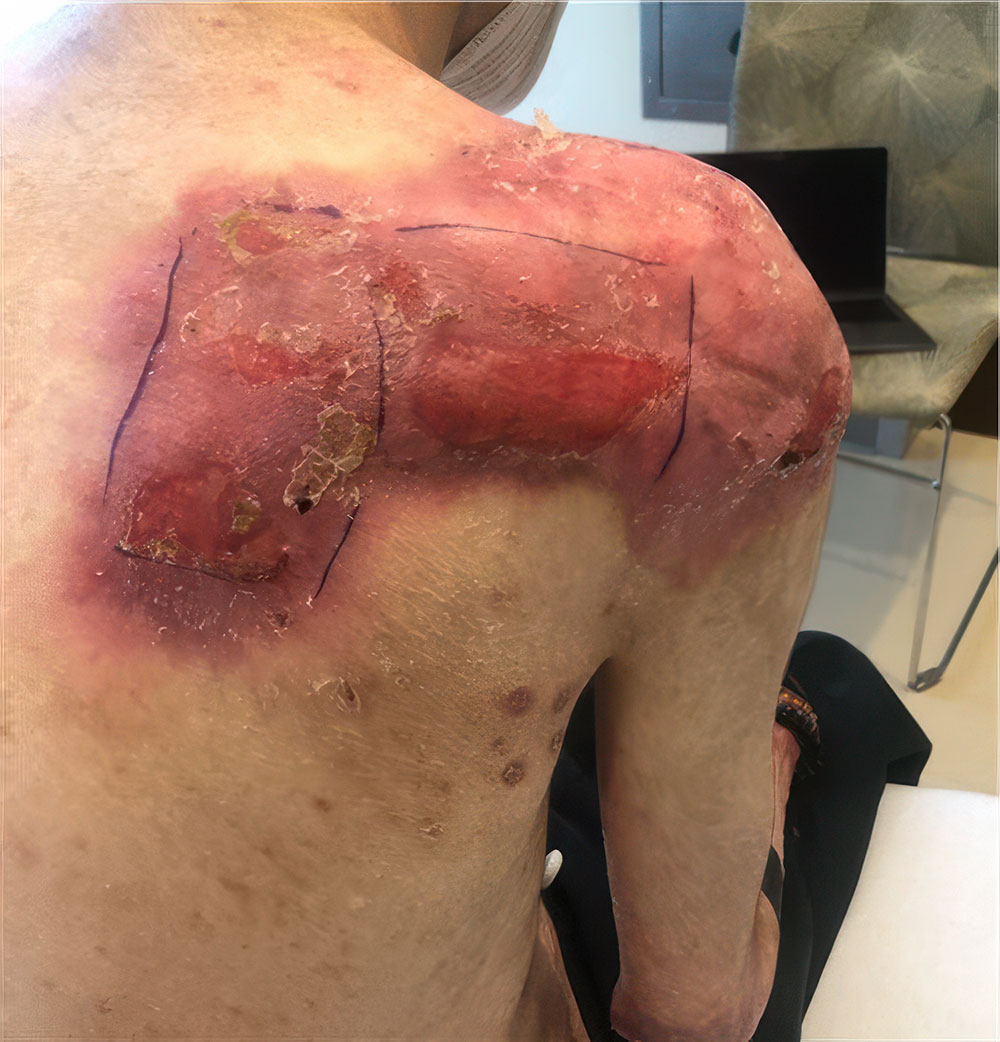
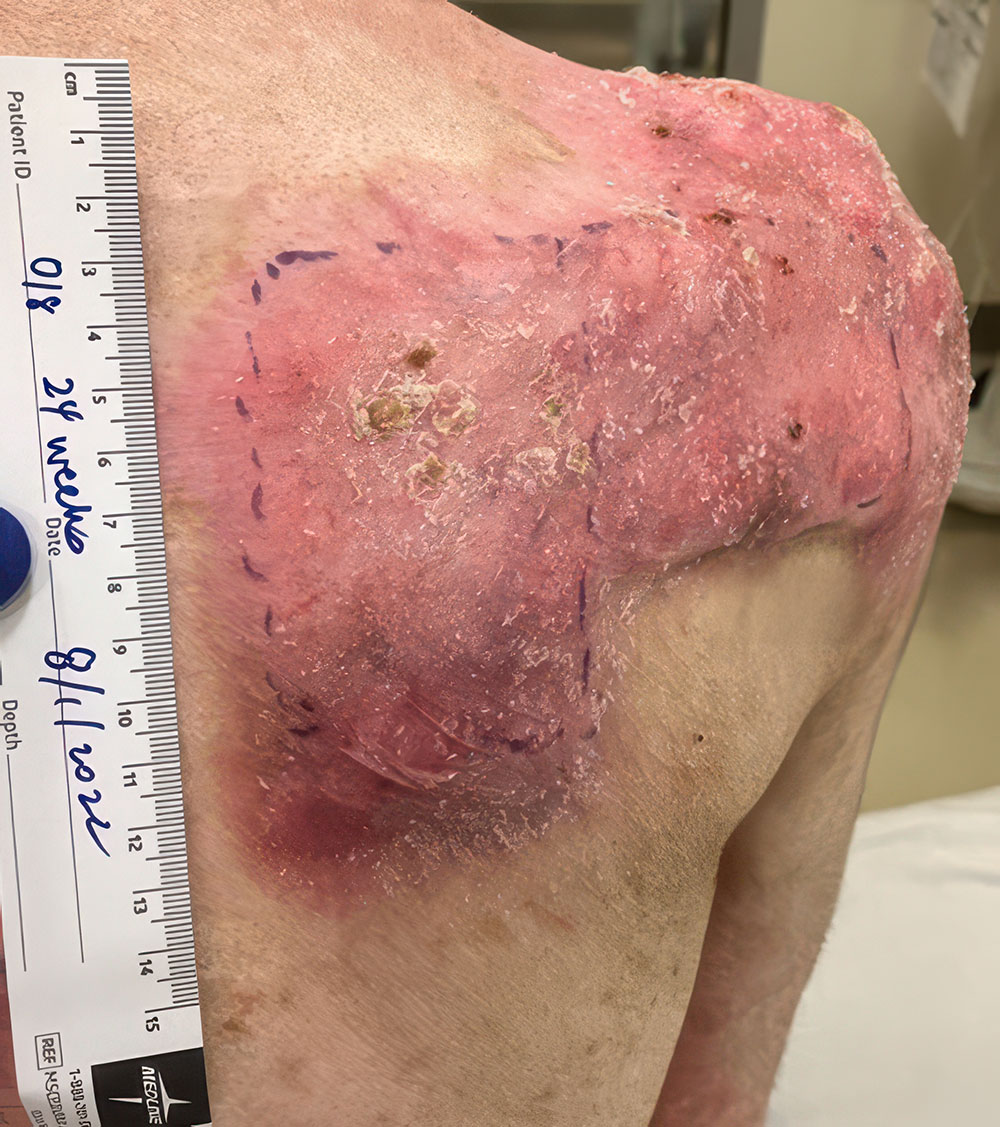
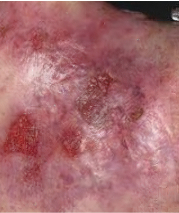
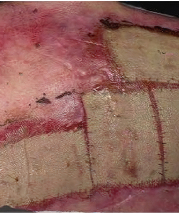
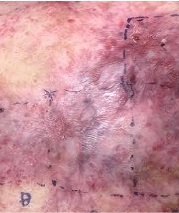
Phase 3 (VIITAL study) | Before and after images
Before and after ZEVASKYN treatment3
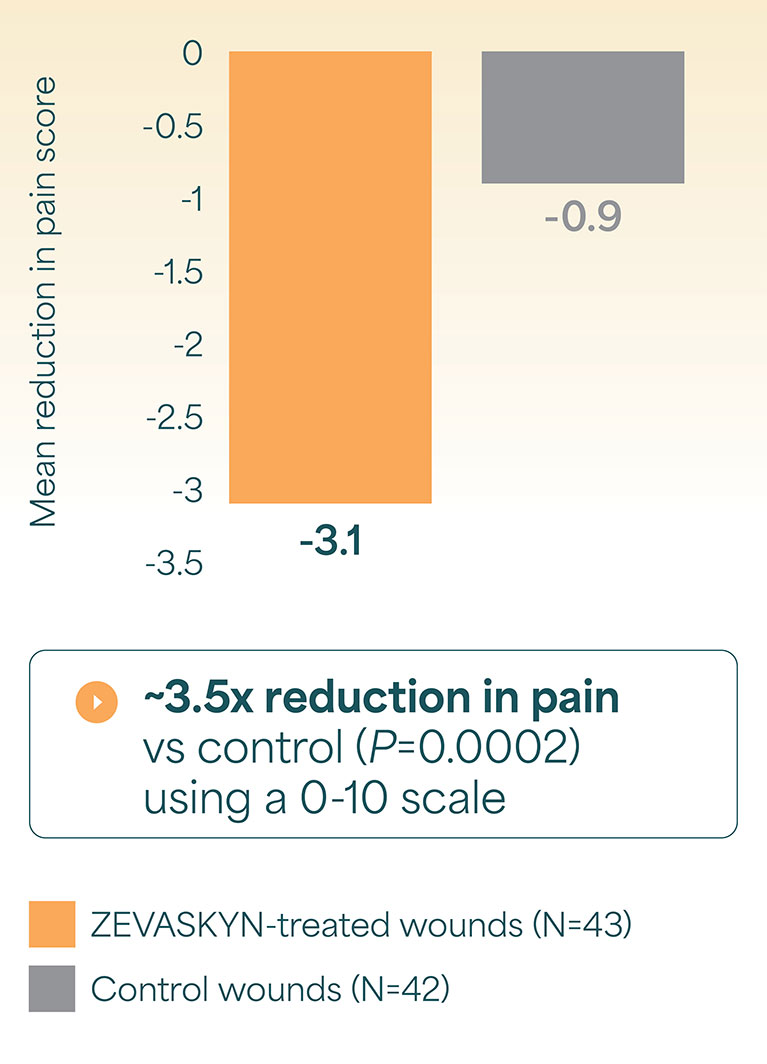
Phase 3 (VIITAL study) | Pain/itch reductions
ZEVASKYN: Proven to reduce pain in treated wounds1,2
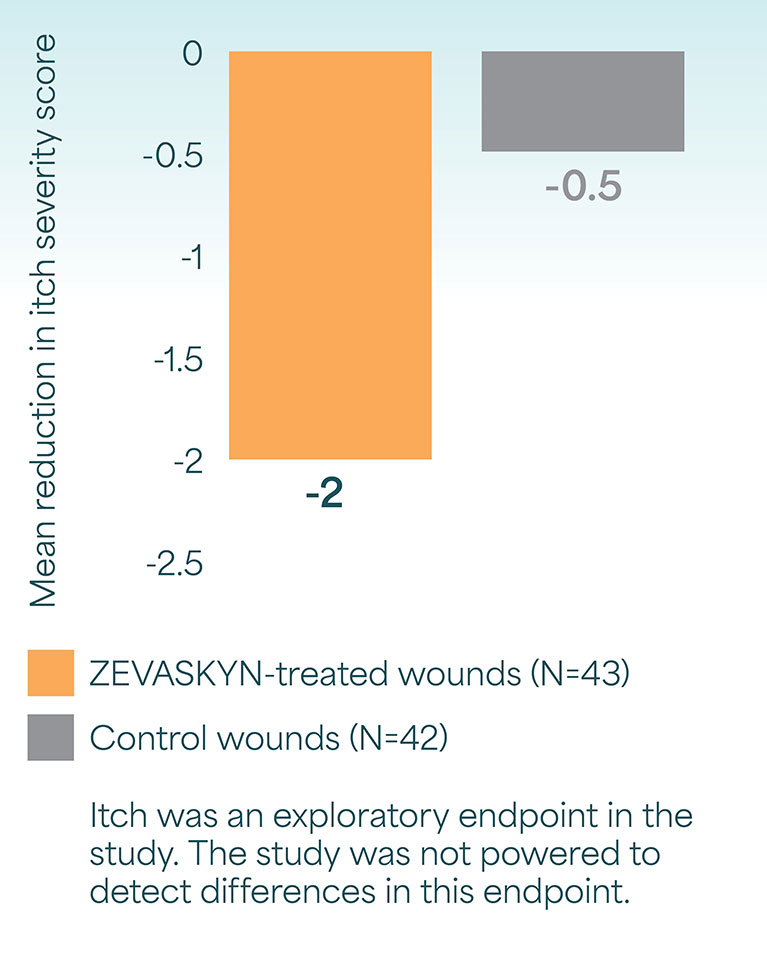
Reduction in pain and itch at week 24 in the VIITAL study
Mean pain reduction from baseline (coprimary endpoint)1¶
Mean reduction in itch severity from baseline2#

Oh, it was bad [before ZEVASKYN was applied]. It was constantly itching, and the medicine wasn’t helping. And then [after ZEVASKYN was applied, there] was less itching…and less pain too. When it heals [after surgery], I don’t feel itching. No more itching, no more pain.”
- N=wounds, n=patients.
- ¶Pain was assessed via the Wong-Baker FACES® scale or numeric rating scale (0-10) following wound dressing change. For every postbaseline assessment, pain reduction is defined as postbaseline pain score minus baseline pain score.1,2
- #Itch severity was assessed using the Worst Itch-Numeric Rating Scale (WI-NRS), ranging from 0 (no itch) to 10 (worst itch imaginable).2
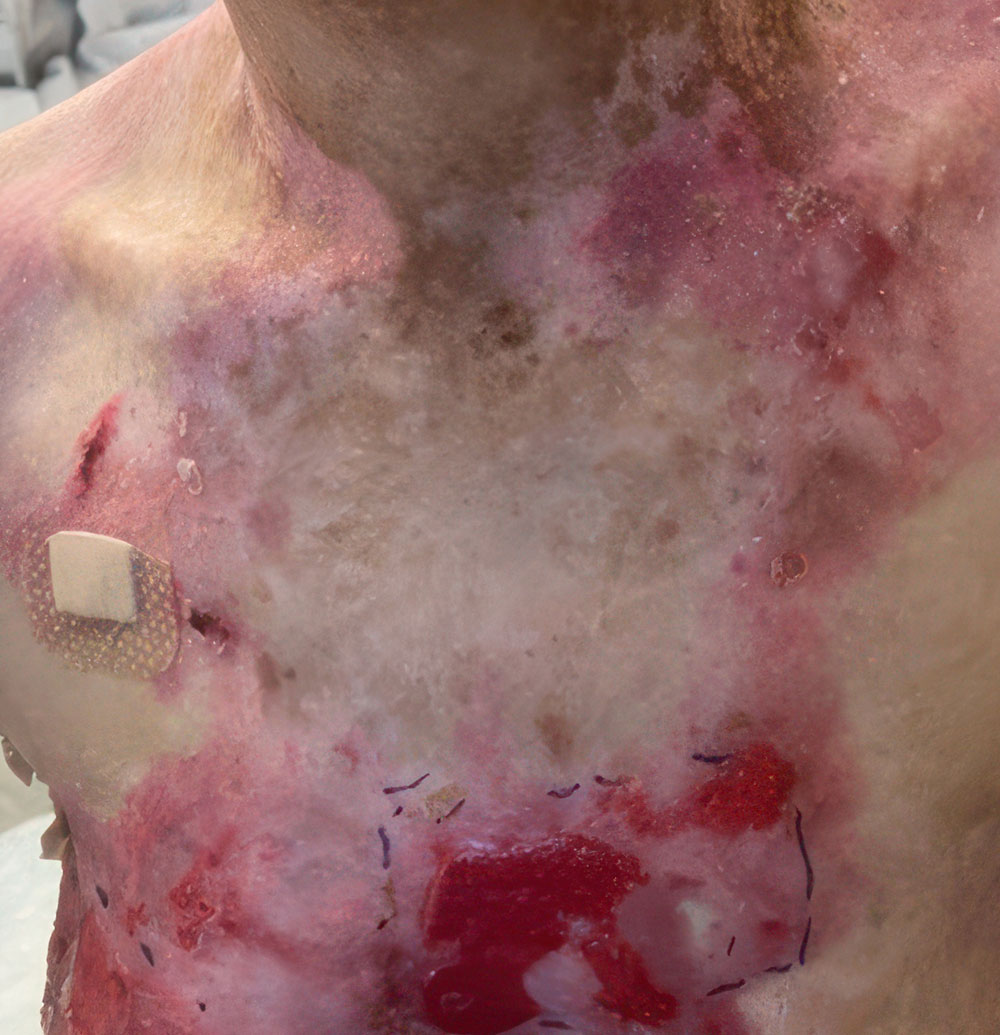
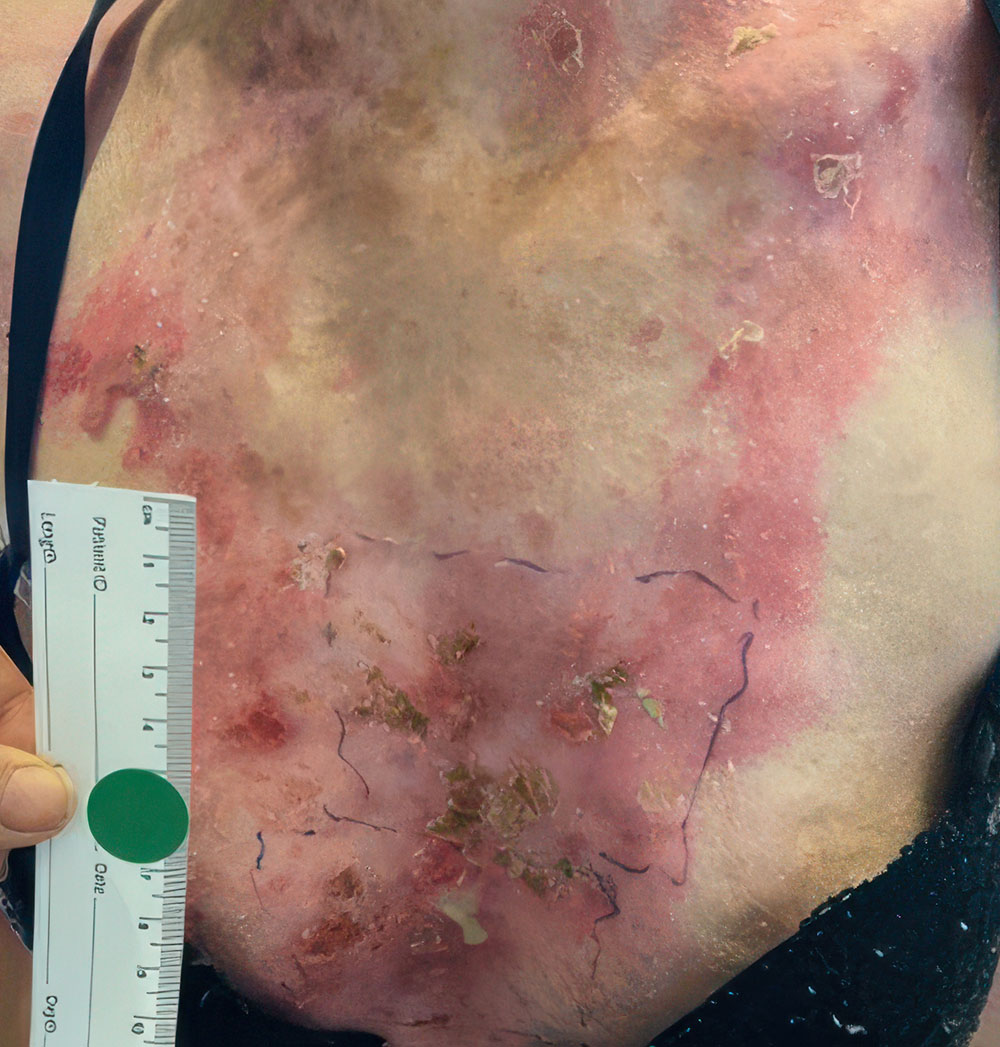
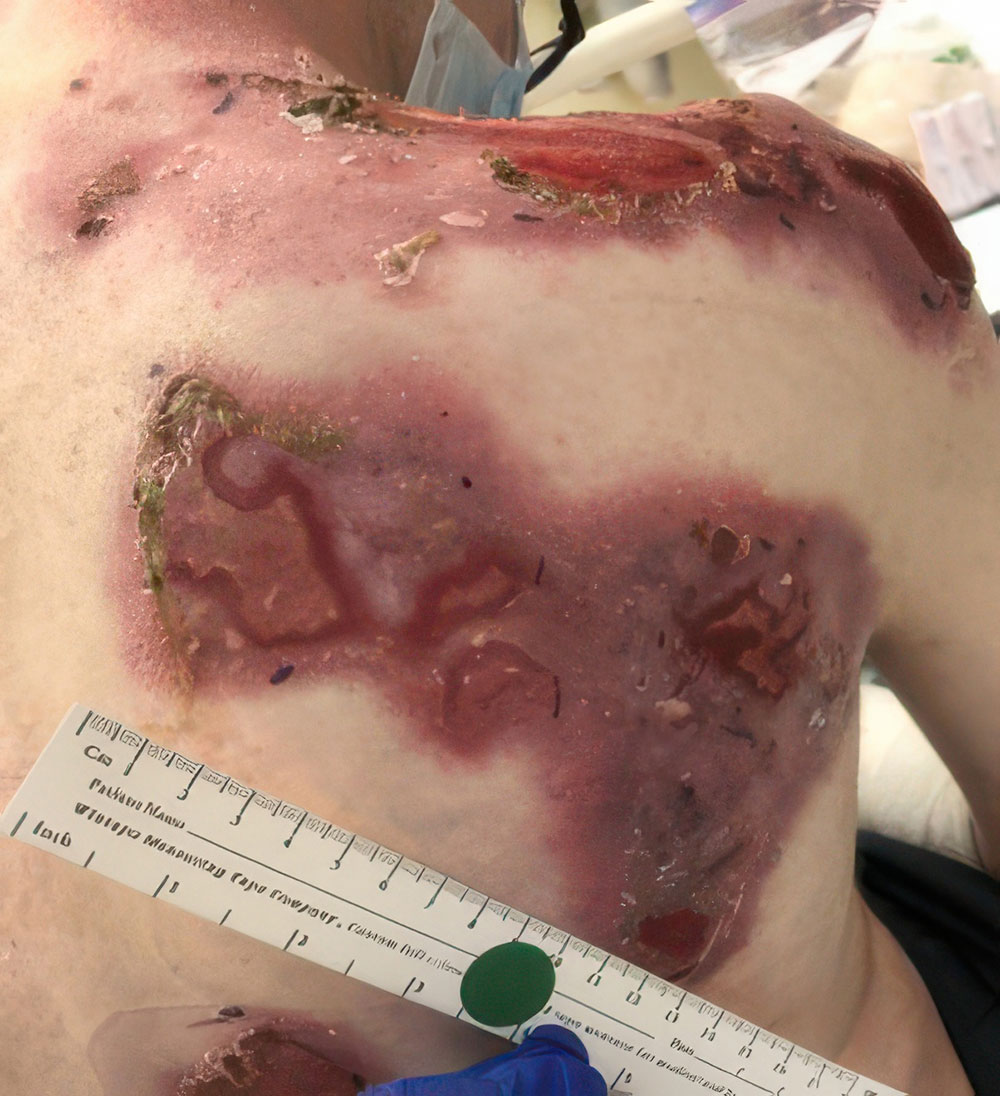
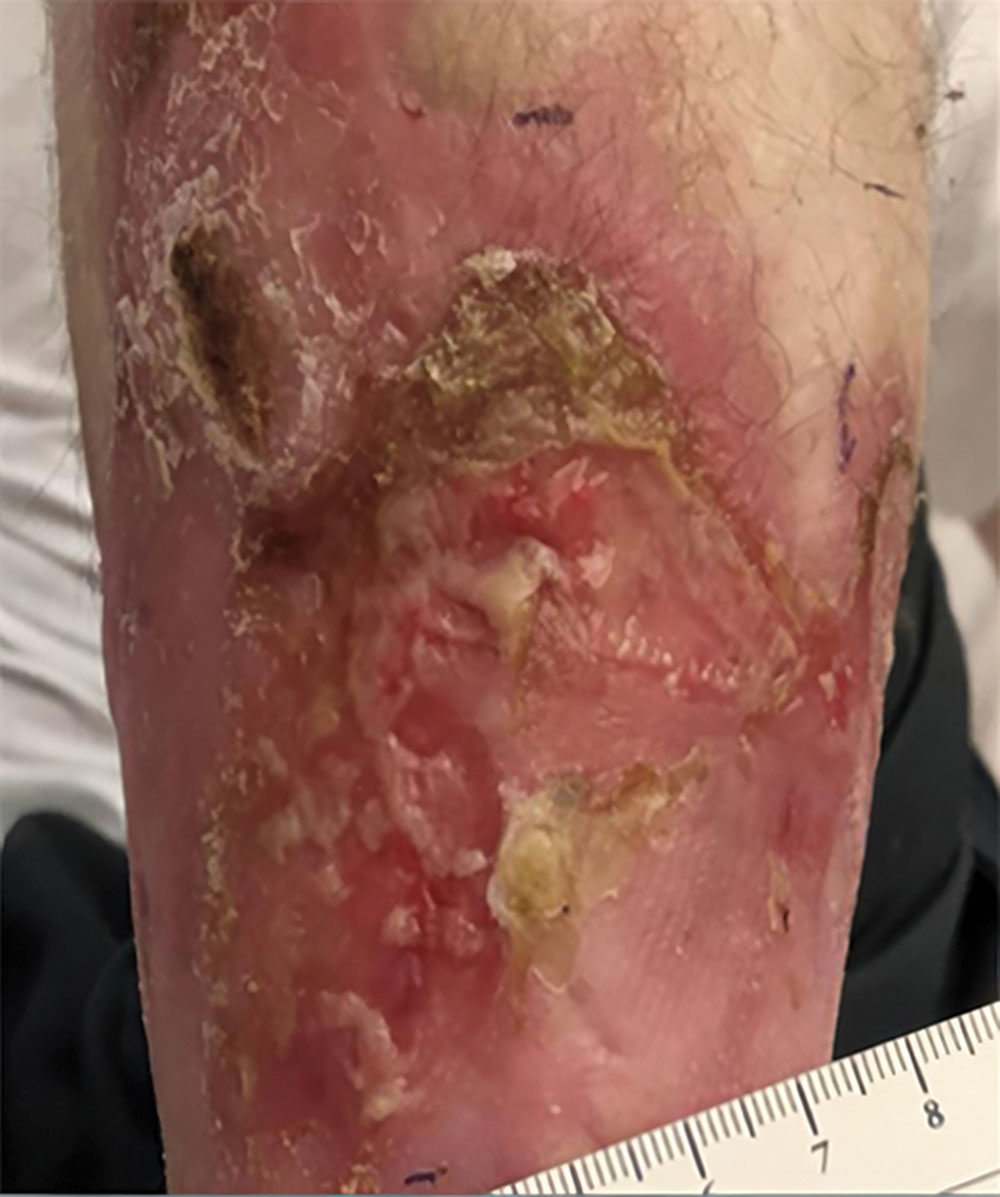
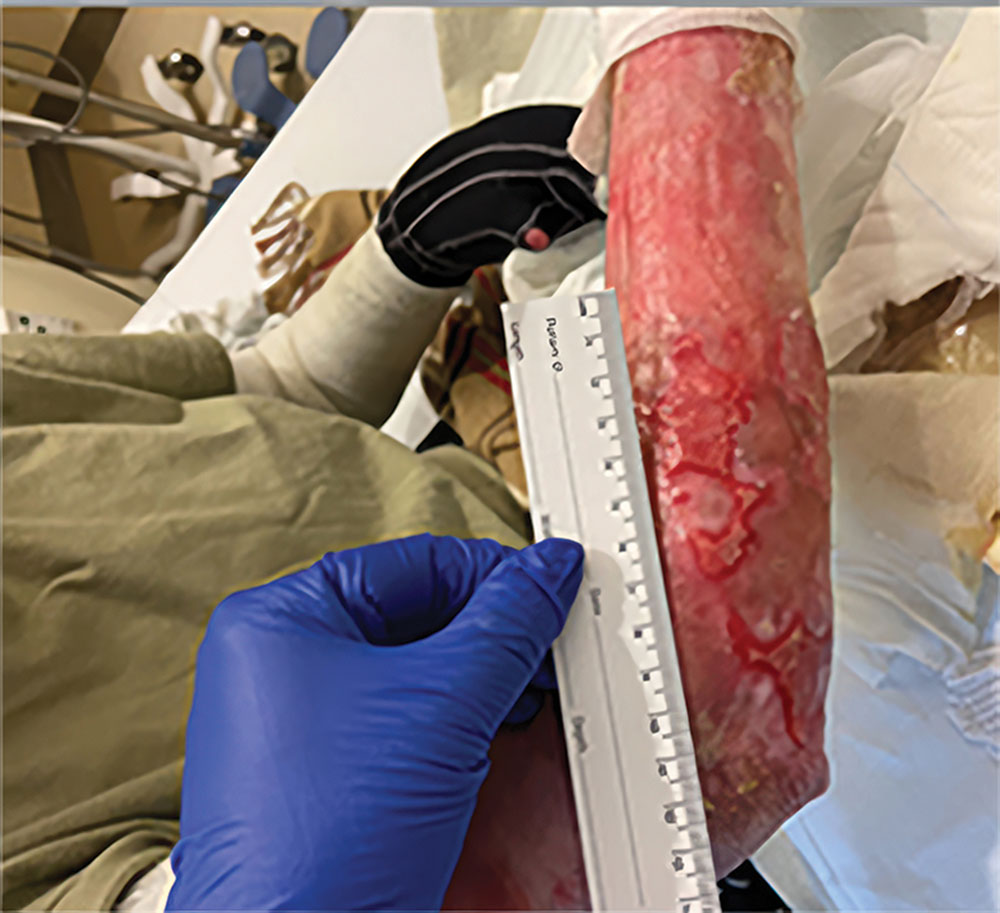
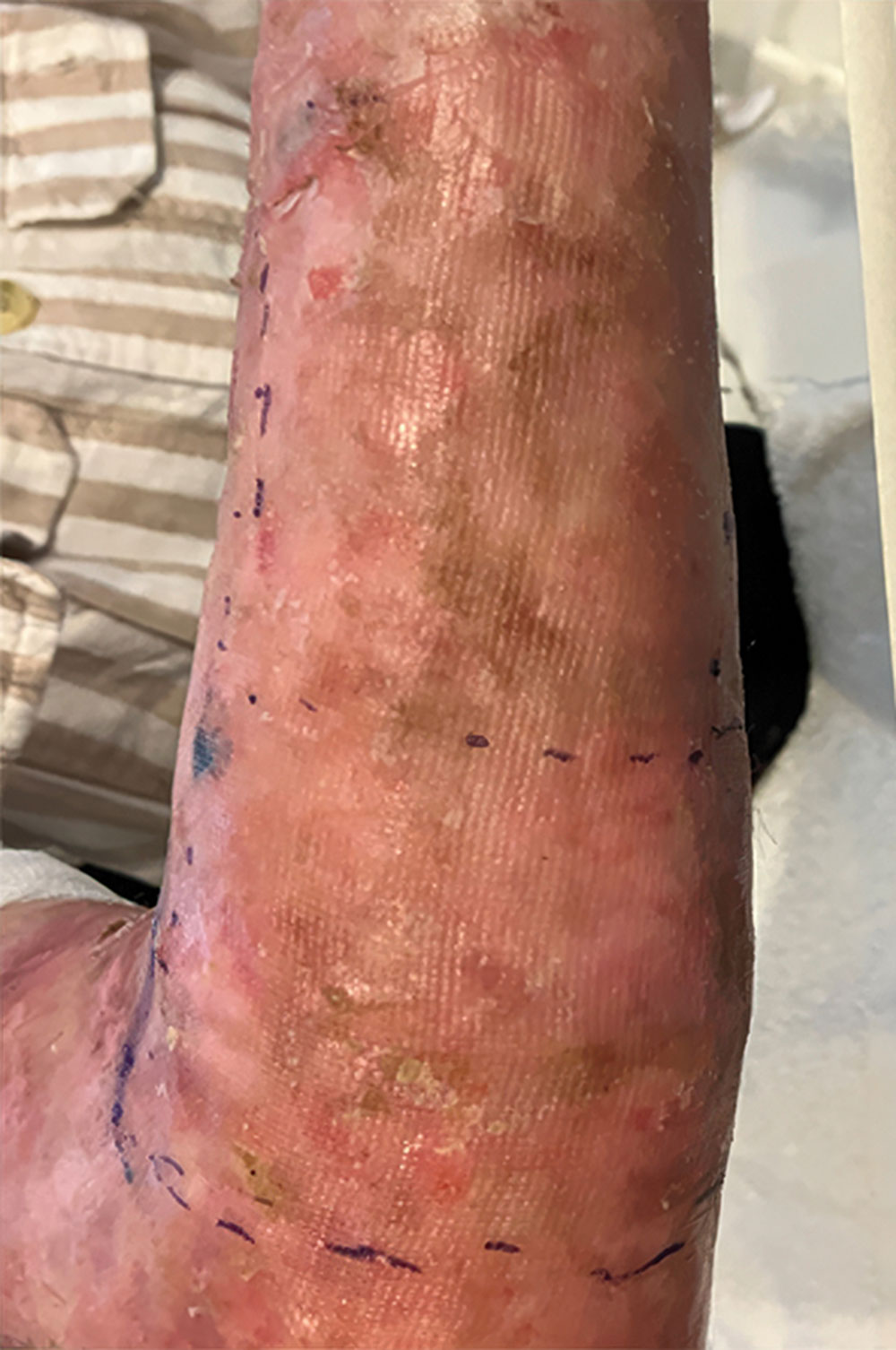
Phase 1/2a long-term follow-up | Long-term follow-up images
Example of wound healing at the end of Phase 1/2a long-term follow-up4
- Study design: Open-label, single-arm, single-center1,5
- Patient population: 7 patients with RDEB; 38 chronic wounds assessed5
- Follow-up duration: Median 6.9 years (range 4-8 years); planned follow-up of 15 years5

So, they were chronic wounds open for 10 plus years with no significant improvement. And so, after the surgery, my skin is much improved, and it doesn’t break down as easy or wound or the area of raw skin is diminished.”